Lucio-Martínez, Fátima and Garda, Zoltán and Váradi, Balázs and Kálmán, Ferenc Krisztián and Esteban-Gómez, David and Tóth, Éva and Tircsó, Gyula and Platas-Iglesias, Carlos (2022) Rigidified Derivative of the Non-macrocyclic Ligand H4OCTAPA for Stable Lanthanide(III) Complexation. INORGANIC CHEMISTRY, 61. pp. 5157-5171. ISSN 0020-1669
|
Text
lucio-martínez-et-al-2022-rigidified-derivative-of-the-non-macrocyclic-ligand-h4octapa-for-stable-lanthanide(iii).pdf - Published Version Available under License Creative Commons Attribution. Download (2MB) | Preview |
|
![[img]](https://real.mtak.hu/205040/7.hassmallThumbnailVersion/graphabs1.jpeg)
|
Text (graphical abstract)
graphabs1.jpeg Available under License Creative Commons Attribution. Download (132kB) | Preview |
Abstract
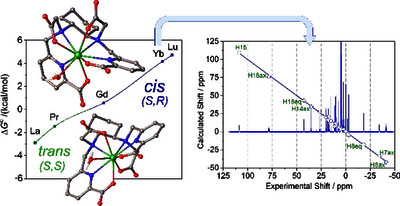
The stability constants of lanthanide complexes with the potentially octadentate ligand CHXOCTAPA4–, which contains a rigid 1,2-diaminocyclohexane scaffold functionalized with two acetate and two picolinate pendant arms, reveal the formation of stable complexes [log KLaL = 17.82(1) and log KYbL = 19.65(1)]. Luminescence studies on the Eu3+ and Tb3+ analogues evidenced rather high emission quantum yields of 3.4 and 11%, respectively. The emission lifetimes recorded in H2O and D2O solutions indicate the presence of a water molecule coordinated to the metal ion. 1H nuclear magnetic relaxation dispersion profiles and 17O NMR chemical shift and relaxation measurements point to a rather low water exchange rate of the coordinated water molecule (kex298 = 1.58 × 106 s–1) and relatively high relaxivities of 5.6 and 4.5 mM–1 s–1 at 20 MHz and 25 and 37 °C, respectively. Density functional theory calculations and analysis of the paramagnetic shifts induced by Yb3+ indicate that the complexes adopt an unprecedented cis geometry with the two picolinate groups situated on the same side of the coordination sphere. Dissociation kinetics experiments were conducted by investigating the exchange reactions of LuL occurring with Cu2+. The results confirmed the beneficial effect of the rigid cyclohexyl group on the inertness of the Lu3+ complex. Complex dissociation occurs following proton- and metal-assisted pathways. The latter is relatively efficient at neutral pH, thanks to the formation of a heterodinuclear hydroxo complex.
| Item Type: | Article |
|---|---|
| Subjects: | Q Science / természettudomány > QD Chemistry / kémia > QD02 Physical chemistry / fizikai kémia Q Science / természettudomány > QD Chemistry / kémia > QD03 Inorganic chemistry / szervetlen kémia |
| Depositing User: | Dr. Ferenc K. Kálmán |
| Date Deposited: | 17 Sep 2024 09:01 |
| Last Modified: | 17 Sep 2024 09:01 |
| URI: | https://real.mtak.hu/id/eprint/205040 |
Actions (login required)
 |
Edit Item |