Gazdag, Tamás and Meiszter, Enikő and Mayer, Péter József and Holczbauer, Tamás and Ottosson, Henrik and Maurer, Andrew B and Abrahamsson, Maria and London, Gábor (2024) An Exploration of Substituent Effects on the Photophysical Properties of Monobenzopentalenes. CHEMPHYSCHEM: A EUROPEAN JOURNAL OF CHEMICAL PHYSICS AND PHYSICAL CHEMISTRY, 25 (7). No. e202300737. ISSN 1439-4235 (In Press)
|
Text
2024_ChemPhysChem.pdf - Published Version Available under License Creative Commons Attribution. Download (2MB) | Preview |
|
![[img]](https://real.mtak.hu/205092/7.hassmallThumbnailVersion/graphical%20abstract.jpg)
|
Text
graphical abstract.jpg - Published Version Available under License Creative Commons Attribution. Download (107kB) | Preview |
Abstract
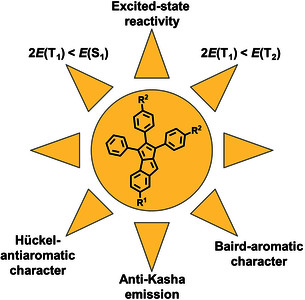
Monobenzopentalenes have received moderate attention compared to dibenzopentalenes, yet their accessibility as stable, non-symmetric structures with diverse substituents could be interesting for materials applications, including molecular photonics. Recently, monobenzopentalene was considered computationally as a potential chromophore for singlet fission (SF) photovoltaics. To advance this compound class towards photonics applications, the excited state energetics must be characterized, computationally and experimentally. In this report we synthesized a series of stable substituted monobenzopentalenes and provided the first experimental exploration of their photophysical properties. Structural and optoelectronic characterization revealed that all derivatives showed 1 H NMR shifts in the olefinic region, bond length alternation in the pentalene unit, low-intensity absorptions reflecting the ground-state antiaromatic character and in turn the symmetry forbidden HOMO-to-LUMO transitions of ~2 eV and redox amphotericity. This was also supported by computed aromaticity indices (NICS, ACID, HOMA). Accordingly, substituents did not affect the fulfilment of the energetic criterion of SF, as the computed excited-state energy levels satisfied the required E(S1)/E(T1)>2 relationship. Further spectroscopic measurements revealed a concentration dependent quenching of the excited state and population of the S2 state on the nanosecond timescale, providing initial evidence for unusual photophysics and an alternative entry point for singlet fission with monobenzopentalenes.
| Item Type: | Article |
|---|---|
| Uncontrolled Keywords: | antiaromaticity · excited state · pentalene · photophysics · singlet fission · substituent effects |
| Subjects: | Q Science / természettudomány > QD Chemistry / kémia > QD02 Physical chemistry / fizikai kémia Q Science / természettudomány > QD Chemistry / kémia > QD04 Organic chemistry / szerves kémia |
| Depositing User: | Gábor London |
| Date Deposited: | 17 Sep 2024 14:13 |
| Last Modified: | 17 Sep 2024 14:13 |
| URI: | https://real.mtak.hu/id/eprint/205092 |
Actions (login required)
 |
Edit Item |