András Dávid, Tóth and Eszter, Soltész-Katona and Katalin, Kis and Viktor, Guti and Sharon, Gilzer and Susanne, Prokop and Roxana, Boros and Ádám, Misák and András, Balla and Péter, Várnai and Lilla, Turiák and András, Ács and László, Drahos and Asuka, Inoue and László, Hunyady and Gábor, Turu (2024) ArreSTick motif controls β-arrestin-binding stability and extends phosphorylation-dependent β-arrestin interactions to non-receptor proteins. CELL REPORTS, 43. No.-114241. ISSN 2211-1247
|
Text
CellReports2023.04.27.538587v1.full.pdf - Published Version Available under License Creative Commons Attribution. Download (4MB) | Preview |
|
![[img]](https://real.mtak.hu/205742/7.hassmallThumbnailVersion/IMG_1181.jpeg)
|
Text (Graphical Abstract)
IMG_1181.jpeg - Other Available under License Creative Commons Attribution. Download (55kB) | Preview |
Abstract
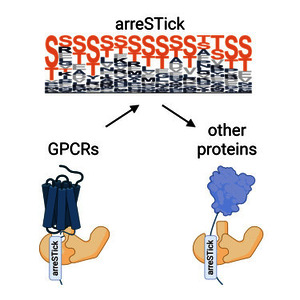
The binding and function of β-arrestins are regulated by specific phosphorylation motifs present in G protein-coupled receptors (GPCRs). However, the exact arrangement of phosphorylated amino acids responsible for establishing a stable interaction remains unclear. We employ a 1D sequence convolution model trained on GPCRs with established β-arrestin-binding properties. With this approach, amino acid motifs characteristic of GPCRs that form stable interactions with β-arrestins can be identified, a pattern that we name “arreSTick.” Intriguingly, the arreSTick pattern is also present in numerous non-receptor proteins. Using proximity biotinylation assay and mass spectrometry analysis, we demonstrate that the arreSTick motif controls the interaction between many non-receptor proteins and β-arrestin2. The HIV-1 Tat-specific factor 1 (HTSF1 or HTATSF1), a nuclear transcription factor, contains the arreSTick pattern, and its subcellular localization is influenced by β-arrestin2. Our findings unveil a broader role for β-arrestins in phosphorylation-dependent interactions, extending beyond GPCRs to encompass non-receptor proteins as well.
| Item Type: | Article |
|---|---|
| Uncontrolled Keywords: | Keywords machine learning convolution phosphorylation GPCR arrestin, arreSTick, proximity biotinylation assay, mass spectrometry, HTATSF1, HTSF1 |
| Subjects: | R Medicine / orvostudomány > R1 Medicine (General) / orvostudomány általában > R850-854 Experimental medicine / kisérleti orvostudomány |
| Depositing User: | Dr. Gábor Turu |
| Date Deposited: | 25 Sep 2024 05:48 |
| Last Modified: | 25 Sep 2024 05:48 |
| URI: | https://real.mtak.hu/id/eprint/205742 |
Actions (login required)
 |
Edit Item |