Inczefi, Orsolya and Eutamene, Hélène and Placide, Fanny and Tondereau, Valérie and Pallagi, Petra and Bagyánszki, Mária and Bódi, Nikolett and Gémes, Nikolett and Szebeni, Gábor and Molnár, Tamás and Theodorou, Vassilia and Róka, Richárd László (2024) Translational evaluation of Gelsectan® effects on gut barrier dysfunction and visceral pain in animal models and irritable bowel syndrome with diarrhoea. UNITED EUROPEAN GASTROENTEROLOGY JOURNAL. ISSN 2050-6406
|
Text
UEGJournal-2024-Inczefi-TranslationalevaluationofGelsectaneffectsongutbarrierdysfunctionandvisceralpain1.pdf - Published Version Available under License Creative Commons Attribution Non-commercial No Derivatives. Download (2MB) | Preview |
|
![[img]](https://real.mtak.hu/205768/7.hassmallThumbnailVersion/ga.jpg)
|
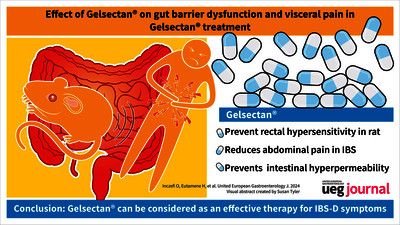
Text (graphical abstract)
ga.jpg - Other Available under License Creative Commons Attribution Non-commercial No Derivatives. Download (580kB) | Preview |
Abstract
Background Gelsectan® is a formulation of xyloglucan (XG), pea protein, grape seed extract (PPGS) and xylo-oligosaccharides (XOS). Our aim was to examine the effect of Gelsectan® on rectal sensitivity in an animal model, abdominal pain in irritable bowel syndrome with diarrhoea (IBS-D) subjects and intestinal permeability in both conditions. Methods Animals: Wistar rats received gavage with XOS, XG + PPGS or XG + PPGS + XOS, as a single dose or for 7 days before a partial restraint stress (PRS). Visceromotor response to rectal distension and total gut paracellular permeability to 51Cr-EDTA were assessed. Humans: IBS-D and control patients were involved. After initial colonoscopy with biopsy sampling Gelsectan® was administered to IBS-D patients for 12 weeks. Stool count and pain scores were documented. After treatment, colonoscopy was repeated. The permeability of biopsy samples was measured in Ussing-chambers. Adherent mucus layer, Muc-2 expression as well as TNFα, Interferon IFNγ were evaluated by histology/immunohistochemistry and ELISA assays, respectively. Results Animal studies: In control rats, PRS significantly increased visceromotor response as well as gut paracellular permeability. Single dose administration of XG + PPGS + XOS failed to reverse PRS, but 7 days of oral treatment reversed PRS-induced rectal hypersensitivity and gut hyperpermeability. Human studies: Gelsectan® treatment significantly reduced and abdominal pain. Intestinal permeability in IBS-D patients was elevated compared with controls, Gelsectan® restored permeability in the ascendent colon. Periodic acid–Schiff-stained mucus layer was significantly thinner in IBS-D patients compared with controls, In both segments, mucus thickness and the proportion of Muc-2 positive cells were not affected by Gelsectan® treatment. IFNγ tissue level in the sigmoid colon shows modest mucosal inflammation in IBS-D. Conclusions Gelsectan® prevented rectal hypersensitivity in rats, abdominal pain in human and intestinal hyperpermeability in rat and human studies respectively. These effects involve restoration of gut permeability. Based on this translational study, Gelsectan® can be considered as an effective therapy for IBS-D symptoms.
| Item Type: | Article |
|---|---|
| Uncontrolled Keywords: | Gelsectan, gut hyperpermeability, irritable bowel syndrome, rectal hypersensitivity |
| Subjects: | R Medicine / orvostudomány > R1 Medicine (General) / orvostudomány általában |
| SWORD Depositor: | MTMT SWORD |
| Depositing User: | MTMT SWORD |
| Date Deposited: | 25 Sep 2024 06:59 |
| Last Modified: | 25 Sep 2024 06:59 |
| URI: | https://real.mtak.hu/id/eprint/205768 |
Actions (login required)
 |
Edit Item |