Kanti, S P Yamini and Pannonhalminé Csóka, Ildikó and Adalbert, Lívia and Jójártné Laczkovich, Orsolya (2022) Analysis of the renewed European Medical Device Regulations in the frame of the non – EU regulatory landscape during the COVID facilitated change. JOURNAL OF PHARMACEUTICAL SCIENCES, 111 (10). pp. 2674-2686. ISSN 0022-3549
|
Text
Article3.pdf - Published Version Restricted to Repository staff only Download (1MB) | Request a copy |
||
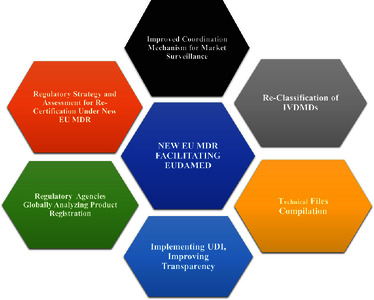
![[img]](https://real.mtak.hu/217020/7.hassmallThumbnailVersion/gra.jpg)
|
Text (graphical abstract)
gra.jpg - Published Version Download (137kB) | Preview |
Abstract
The term “Medical devices” includes technology-based devices or articles, both basic and complex. Due to these types of variations, a strict, robust, transparent, and sustainable regulatory framework is required. In recent clinical practice, incidents including the breast implant and the hip replacement crisis have made it necessary to improve the regulatory and compliance approaches for the industry to ensure the manufacturing and distribution of safe and innovative MDs within the EU. In response to this, the EU revised the laws governing medical devices and in vitro diagnostics to align with the developments of the sector, address critical safety issues and support innovation. The new regulation (EU) 2017/745 on Medical Devices (MDR) is now applicable from May 26 2021 and the In Vitro Diagnostic Medical Devices Regulation (EU) 2017/746 will take effect from May 2022.In this review, we aim to provide an update on the new Medical Device Regulations in the context of the current medical needs of the world, and also to give a glimpse at the non-EU regulatory landscape. Finally, we take a look at the closed-system transfer devices (CSTD) and COVID facilitated changes promoting demand for continuous improvement and trends in the pharmaceutical and medical industry related areas.
| Item Type: | Article |
|---|---|
| Uncontrolled Keywords: | Medical Devices, MD and IVD regulation in Europe, EMEA, USFDA, MDR INDIA, COVID implications, Drug Delivery Systems, Regulatory Science, Closed-System Transfer Devices (CSTD) , Medical devices (MD) , In Vitro Diagnostic (IVD), Medical Device Rules (MDR), European Union (EU), European Standard (EN), international organization for standardization (ISO), international electrotechnical commission (IEC), European Database on Medical Devices (EUDAMED), Unique Device Identification (UDI), Corrective and Preventive Actions (CAPA), Closed System Transfer Devices (CSTDs), Drug Device Combination (DDC), Personal Protective Equipment (PPE), Ethics Committee (EC), Public Health care (PHL), Pre-market Approval (PMA), Code of Federal Regulations (CFR), Food and Drug Administration (FDA) |
| Subjects: | R Medicine / orvostudomány > RM Therapeutics. Pharmacology / terápia, gyógyszertan |
| SWORD Depositor: | MTMT SWORD |
| Depositing User: | MTMT SWORD |
| Date Deposited: | 19 Mar 2025 06:41 |
| Last Modified: | 19 Mar 2025 06:41 |
| URI: | https://real.mtak.hu/id/eprint/217020 |
Actions (login required)
 |
Edit Item |