Szalai, Boglárka and Budai-Szűcs, Mária and Kovács, Anita and Berkó, Szilvia and Gróf, Ilona and Deli, Mária Anna and Katona, Gábor and Balogh, György Tibor and Jójártné Laczkovich, Orsolya (2024) The effect of mucoadhesive polymers on ocular permeation of thermoresponsive in situ gel containing dexamethasone–cyclodextrin complex. INTERNATIONAL JOURNAL OF PHARMACEUTICS, 667 (A). ISSN 0378-5173
|
Text
SzalaiB_TheEffect.pdf - Published Version Available under License Creative Commons Attribution Non-commercial No Derivatives. Download (4MB) | Preview |
|
![[img]](https://real.mtak.hu/217857/7.hassmallThumbnailVersion/1-s2.0-S0378517324010822-ga1_lrg.jpg)
|
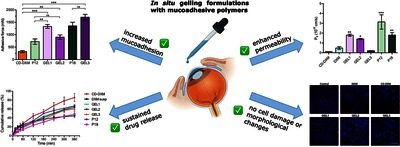
Text (graphical abstract)
1-s2.0-S0378517324010822-ga1_lrg.jpg - Published Version Available under License Creative Commons Attribution Non-commercial No Derivatives. Download (238kB) | Preview |
Abstract
Dexamethasone (DXM) is a commonly used corticosteroid in the treatment of ocular inflammatory conditions that affect more and more people. The aim of this study was to evaluate the effect of the combination of hydroxypropyl-β-cyclodextrin (HPBCD), in situ gelling formulations, and other mucoadhesive polymers, i.e., hydroxypropyl methylcellulose (HPMC) and zinc-hyaluronate (ZnHA), on permeation by applying in vitro and ex vivo ophthalmic permeation models. Additionally, gelling properties, in vitro drug release, and mucoadhesion were measured to determine the impact of these factors on permeation and ultimately on bioavailability. The results showed that GEL1 and GEL2 had an optimal gelling temperature, 36.3 ℃ and 34.6 ℃, respectively. Moreover, the combination of Poloxamer 407 (P407) with other polymers improved the mucoadhesion (GEL1: 1333.7 mN) compared with formulations containing only P407 (P12: 721.8 mN). Both HPBCD and the gel matrix had a considerable influence on the drug release and permeability of DXM, and the combination could facilitate the permeation into the aqueous humor. After 30 min of treatment, the DXM concentration in the aqueous humor was 1.16–1.37 µg∕mL in case of the gels, whereas DXM could not be detected when treated with the DXM suspension. The results of the experiments using an in vitro cell line indicated that the formulations could be considered safe for topical treatment of the eye. In conclusion, with application of a small amount of HPMC (0.2 % w∕w), the concentration of P407 could be reduced to 12 % w/w while maintaining the ideal gelling properties and gel structure without negatively affecting permeability compared with the formulation containing a higher amount of P407. Furthermore, the gel matrix may also provide programmed and elongated drug release.
| Item Type: | Article |
|---|---|
| Additional Information: | Cyclodextrin, In situ gelling, Ophthalmic drug delivery, Permeation, Poloxamer |
| Subjects: | R Medicine / orvostudomány > RM Therapeutics. Pharmacology / terápia, gyógyszertan |
| SWORD Depositor: | MTMT SWORD |
| Depositing User: | MTMT SWORD |
| Date Deposited: | 16 Apr 2025 05:23 |
| Last Modified: | 16 Apr 2025 05:23 |
| URI: | https://real.mtak.hu/id/eprint/217857 |
Actions (login required)
 |
Edit Item |