Bizet, Maxime and Balázsi, Áron and Biaso, Frédéric and Byrne, Deborah and Etienne, Emilien and Guigliarelli, Bruno and Urban, Philippe and Dorlet, Pierre and Truan, Gilles and Gerbaud, Guillaume and Kálai, Tamás and Martinho, Marlène (2025) Expanding the Diversity of Nitroxide-Based Paramagnetic Probes Conjugated to Non-Canonical Amino Acids for Sdsl-Epr Applications. CHEMBIOCHEM, 26. No. e202500064. ISSN 1439-4227
|
Text
ChemBioChem - 2025 - Bizet - Expanding the Diversity of Nitroxide‐Based Paramagnetic Probes Conjugated to Non‐Canonical (3).pdf - Published Version Available under License Creative Commons Attribution Non-commercial No Derivatives. Download (1MB) | Preview |
|
![[img]](https://real.mtak.hu/218247/7.hassmallThumbnailVersion/graphical_abstract.jpg)
|
Text
graphical_abstract.jpg - Published Version Available under License Creative Commons Attribution Non-commercial No Derivatives. Download (393kB) | Preview |
Abstract
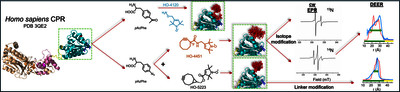
Understanding protein structure requires studying its dynamics, which is critical to elucidating its functional role. Biophysical techniques have revolutionized this field over time, providing remarkable insights into structure-function relationships. Among these, Site-Directed Spin Labelling (SDSL) combined with Electron Paramagnetic Resonance (EPR) is a powerful method delivering structural data at the residue level, irrespective of protein size or environment. Traditional nitroxide labels targeting cysteine residues face limitations when these residues are essential for protein structure or function. To address this, alternatives have been proposed as the use of non-canonical amino acids (ncaa) coupled with specific nitroxide labels. This study introduces 14N-HO-5223, a novel nitroxide label specific to the pAzPhe ncaa, along with its 15N-derivative. These labels were grafted at two sites of the model protein, the diflavin cytochrome P450 reductase. For comparative purpose, two already reported labels were also used. Continuous wave (cw) EPR spectroscopy confirmed the HO-5223 label as an effective reporter of protein dynamics. Additionally, Double Electron Electron Resonance (DEER) measurements provided distance distributions between the semi-quinone FMNH* state of the CPR and all nitroxide labels. These results expand the toolkit of the ncaa-nitroxide pairs, enabling EPR-based structural studies of proteins where cysteine modification is impractical, further advancing our ability to decode protein dynamics and function.
| Item Type: | Article |
|---|---|
| Subjects: | Q Science / természettudomány > QD Chemistry / kémia > QD04 Organic chemistry / szerves kémia |
| Depositing User: | Dr. Tamás Kálai |
| Date Deposited: | 24 Apr 2025 07:23 |
| Last Modified: | 24 Apr 2025 07:23 |
| URI: | https://real.mtak.hu/id/eprint/218247 |
Actions (login required)
 |
Edit Item |