Semghouli, Anas and Drahos, László and Jianlin, Han and Kiss, Loránd and Nonn, Melinda (2025) Selective Synthesis of Tetrahydroisoquinoline and Piperidine Scaffolds by Oxidative Ring Opening/Ring Closing Protocols of Substituted Indenes and Cyclopentenes. CHEMISTYOPEN. ISSN 2191-1363
|
Text
ChemistryOpen - 2024 - Semghouli - Selective Synthesis of Tetrahydroisoquinoline and Piperidine Scaffolds by Oxidative Ring.pdf - Published Version Available under License Creative Commons Attribution. Download (5MB) | Preview |
|
![[img]](https://real.mtak.hu/218839/7.hassmallThumbnailVersion/open202400475-toc-0001-m.jpg)
|
Text
open202400475-toc-0001-m.jpg - Published Version Available under License Creative Commons Attribution. Download (268kB) | Preview |
Abstract
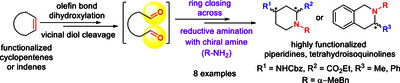
Novel tetrahydroisoquinoline and piperidine derivatives were selectively synthesized from substituted indenes or cyclopentenes. The process starts with an oxidative cleavage of the ring olefin bond, which gives reactive diformyl intermediates. By a ring-closing step using chiral (R) or (S) α-methylbenzylamine under a reductive amination protocol facilitated ring formation with ring expansion of the corresponding nitrogen-containing heterocycles. The stereocontrolled methodology enabled accurate control of the stereochemistry of the final products. Additionally, the synthesized amino acid derivatives possessing an aryl moiety in their structure may be relevant building blocks for foldamer chemistry.
| Item Type: | Article |
|---|---|
| Subjects: | Q Science / természettudomány > QD Chemistry / kémia > QD04 Organic chemistry / szerves kémia |
| Depositing User: | Dr Loránd Kiss |
| Date Deposited: | 14 May 2025 09:34 |
| Last Modified: | 14 May 2025 09:34 |
| URI: | https://real.mtak.hu/id/eprint/218839 |
Actions (login required)
 |
Edit Item |