Keeley, Aaron Brian and Kopranovic, Aleksandra and Di Lorenzo, Vincenzo and Ábrányi-Balogh, Péter and Jänsch, Niklas and Lai, Linh N. and Petri, László and Orgován, Zoltán and Pölöske, Daniel and Orlova, Anna and Németh, András György and Desczyk, Charlotte and Imre, Timea and Bajusz, Dávid and Moriggl, Richard and Meyer-Almes, Franz-Josef and Keserű, György Miklós (2024) Electrophilic MiniFrags Revealed Unprecedented Binding Sites for Covalent HDAC8 Inhibitors. JOURNAL OF MEDICINAL CHEMISTRY, 67 (1). pp. 572-585. ISSN 0022-2623
|
Text
keeley-et-al-2023-electrophilic-minifrags-revealed-unprecedented-binding-sites-for-covalent-hdac8-inhibitors.pdf - Published Version Available under License Creative Commons Attribution. Download (6MB) | Preview |
|
![[img]](https://real.mtak.hu/224479/7.hassmallThumbnailVersion/images_large_jm3c01779_0011.jpeg)
|
Text (graphical abstract)
images_large_jm3c01779_0011.jpeg - Published Version Available under License Creative Commons Attribution. Download (127kB) | Preview |
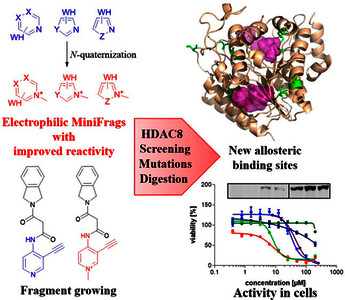
Abstract
Screening of ultra-low-molecular weight ligands (MiniFrags) successfully identified viable chemical starting points for a variety of drug targets. Here we report the electrophilic analogues of MiniFrags that allow the mapping of potential binding sites for covalent inhibitors by biochemical screening and mass spectrometry. Small electrophilic heterocycles and their N-quaternized analogues were first characterized in the glutathione assay to analyze their electrophilic reactivity. Next, the library was used for systematic mapping of potential covalent binding sites available in human histone deacetylase 8 (HDAC8). The covalent labeling of HDAC8 cysteines has been proven by tandem mass spectrometry measurements, and the observations were explained by mutating HDAC8 cysteines. As a result, screening of electrophilic MiniFrags identified three potential binding sites suitable for the development of allosteric covalent HDAC8 inhibitors. One of the hit fragments was merged with a known HDAC8 inhibitor fragment using different linkers, and the linker length was optimized to result in a lead-like covalent inhibitor. © 2023 The Authors. Published by American Chemical Society
| Item Type: | Article |
|---|---|
| Additional Information: | Medicinal Chemistry Research Group, Research Centre for Natural Sciences, Magyar tudósok krt 2, Budapest, H-1117, Hungary Department of Organic Chemistry and Technology, Faculty of Chemical Technology and Biotechnology, Budapest University of Technology and Economics, Müegyetem rkp. 3., Budapest, H-1111, Hungary National Laboratory for Drug Research and Development, Budapest, H-1117, Hungary Department of Chemical Engineering and Biotechnology, University of Applied Sciences Darmstadt, Haardtring 100, Darmstadt, 64295, Germany Institute of Animal Breeding and Genetics, University of Veterinary Medicine, Vienna, 1210, Austria MS Metabolomics Research Group, Research Centre for Natural Sciences, Magyar tudósok krt 2, Budapest, H-1117, Hungary Export Date: 14 March 2024 CODEN: JMCMA Correspondence Address: Meyer-Almes, F.-J.; Department of Chemical Engineering and Biotechnology, Haardtring 100, Germany; email: franz-josef.meyer-almes@h-da.de Correspondence Address: Keserü, G.M.; Medicinal Chemistry Research Group, Magyar tudósok krt 2, Hungary; email: keseru.gyorgy@ttk.hu |
| Subjects: | Q Science / természettudomány > QD Chemistry / kémia |
| SWORD Depositor: | MTMT SWORD |
| Depositing User: | MTMT SWORD |
| Date Deposited: | 17 Sep 2025 19:29 |
| Last Modified: | 17 Sep 2025 19:29 |
| URI: | https://real.mtak.hu/id/eprint/224479 |
Actions (login required)
 |
Edit Item |