Záhonyi, Petra and Fekete, Dániel and Moharos, Erzsébet and Nagy, Zsombor Kristóf and Szabó, Edina (2025) In-line Raman-based quantification of the anhydrous content in a fully integrated continuous powder-to-granule line. INTERNATIONAL JOURNAL OF PHARMACEUTICS, 682. No.-125995. ISSN 0378-5173
|
Text
1-s2.0-S0378517325008324-main.pdf - Published Version Available under License Creative Commons Attribution. Download (5MB) | Preview |
|
![[img]](https://real.mtak.hu/224995/7.hassmallThumbnailVersion/1-s2.0-S0378517325008324-ga1_lrg.jpg)
|
Text (graphical abstract)
1-s2.0-S0378517325008324-ga1_lrg.jpg - Published Version Available under License Creative Commons Attribution. Download (260kB) | Preview |
Abstract
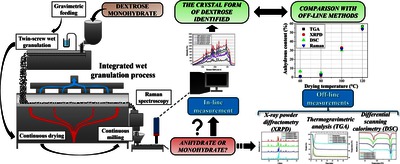
This research is based on a fully integrated continuous twin-screw wet granulation line from powder feeding to dried granules. One of its crucial steps is continuous drying, which can influence the crystal form and thus, the quality of the granules. Therefore, the main objective of this research was to develop a Raman spectroscopic method for monitoring the crystal form transition in-line and real-time during the continuous process while applying different drying temperatures. Dextrose was used as a model material due to its high industrial relevance as an active pharmaceutical ingredient and excipient. The real-time monitoring of the granules’ crystal form demonstrated that drying at higher temperatures (100 °C and 120 °C) resulted in partial dehydration of the initial α-d-glucose monohydrate. Raman spectroscopy combined with multivariate data analysis proved suitable for real-time quantification of anhydrous content with a root mean square error of 3.3 % w/w. The results were validated by various conventional, off-line analytical techniques, which further confirmed the reliability of the model and the detected anhydrous content. This work shows the high predictive performance of Raman spectroscopy for in-line and real-time anhydrous content monitoring in a fully integrated continuous powder-to-granule line, enhancing process understanding and ensuring high and consistent product quality.
| Item Type: | Article |
|---|---|
| Additional Information: | Project no. RRF-2.3.1-21-2022-00015 has been implemented with the support provided by the European Union. This paper was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Science and the Doctoral Excellence Fellowship Programme (DKÖP-25-1-BME-43) funded by the National Research Development and Innovation Fund of the Ministry of Culture and Innovation and the Budapest University of Technology and Economics. This research was supported by the EKÖP-24-3-BME-313 New National Excellence Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund. The scientific research publicized in this article was reached with the sponsorship of Gedeon Richter Talentum Foundation in framework of Gedeon Richter Excellence PhD Scholarship of Gedeon Richter. The authors would like to express their gratitude to Erzsébet Tóth for her help with the DSC, TGA, and XRPD measurements. |
| Uncontrolled Keywords: | Twin-screw granulation, Continuous processing, Solvatomorphs, Process analytical technology (PAT), Hydrates |
| Subjects: | R Medicine / orvostudomány > RM Therapeutics. Pharmacology / terápia, gyógyszertan |
| SWORD Depositor: | MTMT SWORD |
| Depositing User: | MTMT SWORD |
| Date Deposited: | 23 Sep 2025 13:13 |
| Last Modified: | 23 Sep 2025 13:13 |
| URI: | https://real.mtak.hu/id/eprint/224995 |
Actions (login required)
 |
Edit Item |