Dömötör, Orsolya and Pivarcsik, Tamás and Yazdi, Zeinab Nezafat and Bakos, Éva and Laczka, Csilla and Hetényi, Anasztázia and Martinek, Tamás and Szatmári, István and Tóth, Szilárd and Szakács, Gergely and Borics, Attila and Enyedy, Éva Anna (2025) Comparative study of multidrug resistance-targeting 8-hydroxyquinoline-amino acid conjugates: anticancer effect, interaction with human serum albumin and organic anion transporting polypeptides. EUROPEAN JOURNAL OF PHARMACEUTICAL SCIENCES, 212. No. 107187. ISSN 0928-0987
|
Text
1-s2.0-S0928098725001861-main.pdf - Published Version Available under License Creative Commons Attribution Non-commercial No Derivatives. Download (5MB) | Preview |
|
![[img]](https://real.mtak.hu/229586/7.hassmallThumbnailVersion/1-s2.0-S0928098725001861-ga1_lrg.jpg)
|
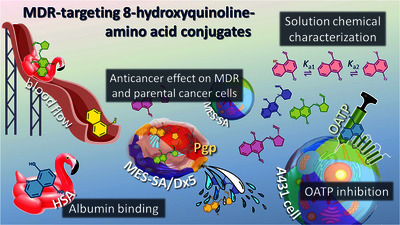
Text (graphical abstract)
1-s2.0-S0928098725001861-ga1_lrg.jpg - Published Version Available under License Creative Commons Attribution Non-commercial No Derivatives. Download (387kB) | Preview |
Abstract
There is a significant demand for the development of targeted and effective treatments for drug-resistant cancer. We have formerly developed several 8-hydroxyquinoline Mannich base compounds whose toxicity is actually enhanced, rather than diminished on multidrug resistant cancer cells. The scope of our work is to provide comprehensive data on the cytotoxicity of D/L-5-chloro-or 5-nitro substituted 7-((homo)proline-1-ylmethyl) quinolin-8-ol compounds on cocultured parental (MES-SA) and ABCB1-expressing MDR (MES-SA/Dx5 and MES-SA/B1) uterine sarcoma cancer cells, to determine the inhibitory effect on organic anion transporting poly-peptides (OATPs) OATP1B1 and OATP2B1, and to characterize the solution chemical and human serum albumin (HSA) binding properties of these molecules, thus assessing certain main pharmacokinetic descriptors in absorption and distribution. Although, 5-chloro substituted amino acid conjugates of the Mannich base 8-hydrox-yquinoline (HQ) derivatives showed moderate cytotoxicity against the parental cells, their anticancer activity was increased on the MDR cell lines. Proton dissociation constants and distribution coefficients were determined and compared; and HSA binding of these molecules was followed by the combination of UV-vis spectrophotometry, time-resolved and steady state fluorometry, circular dichroism spectroscopy, saturation transfer difference NMR and ultrafiltration. HSA serves as a potential carrier for these amino acid conjugates, with at least two binding sites. The inhibition of OATP1B1 and OATP2B1 was studied in vitro and by molecular docking to get an insight on the possible location and mode of binding of these HQ derivatives. Except for the homoproline derivative, the amino acid conjugates were found to be weak inhibitors of these transporters.
| Item Type: | Article |
|---|---|
| Additional Information: | Department of Molecular and Analytical Chemistry, Interdisciplinary Excellence Centre, University of Szeged, Dóm tér 7-8, Szeged, H-6720, Hungary Institute of Molecular Life Sciences, RCNS, Eötvös Loránd Research Network, Magyar tudósok krt. 2, Budapest, H-1117, Hungary Department of Medical Chemistry, University of Szeged, Dóm tér 8, Szeged, Hungary HUN-REN-SZTE Biomimetic Systems Research Group, Dóm tér 8, Szeged, Hungary Institute of Pharmaceutical Chemistry, University of Szeged, Eötvös u. 6, Szeged, H-6720, Hungary HUN REN-SZTE Stereochemistry Research Group, University of Szeged, Eötvös u. 6, Szeged, H-6720, Hungary Drug Resistance Research Group, Institute of Molecular Life Sciences, HUN-REN Research Centre for Natural Sciences, Magyar tudósok krt. 2, Budapest, H-1117, Hungary National Laboratory for Drug Research and Development, Magyar tudósok krt. 2, Budapest, H-1117, Hungary Center for Cancer Research, Medical University of Vienna, Borschkegasse 8a, Vienna, A-1090, Austria Laboratory of Chemical Biology, Institute of Biochemistry, HUN-REN Biological Research Centre, Szeged, Hungary Export Date: 22 July 2025; Cited By: 0; Correspondence Address: O. Dömötör; Department of Molecular and Analytical Chemistry, Interdisciplinary Excellence Centre, University of Szeged, Szeged, Dóm tér 7-8, H-6720, Hungary; email: domotor.o@chem.u-szeged.hu; CODEN: EPSCE |
| Uncontrolled Keywords: | 8-hydroxyquinoline, Cytotoxicity, Multidrug resistance, Albumin binding, Proton dissociation, Lipophilicity |
| Subjects: | R Medicine / orvostudomány > RS Pharmacy and materia medica / gyógyszerészet, gyógyászati eszközök |
| SWORD Depositor: | MTMT SWORD |
| Depositing User: | MTMT SWORD |
| Date Deposited: | 21 Nov 2025 17:50 |
| Last Modified: | 21 Nov 2025 17:50 |
| URI: | https://real.mtak.hu/id/eprint/229586 |
Actions (login required)
 |
Edit Item |