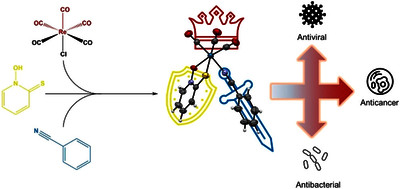
Rapuš, Uroš and Pivarcsik, Tamás and Mitrović, Ana and Kljun, Jakob and Bogdanov, Anita and Spengler, Gabriella and Meden, Anže and Gobec, Stanislav and Kos, Janko and Enyedy, Éva Anna and Turel, Iztok (2025) Synthesis of a fac -Tricarbonylrhenium(I) Complex with Pyrithione, Its Physicochemical Characterization, and Assessment of Biological Effects. ACS OMEGA, 10 (33). pp. 38272-38291. ISSN 2470-1343
|
Text
synthesis-of-a-fac-tricarbonylrheniumi-complex-with-pyrithione-its-physicochemical-characterization-and-assessment-of.pdf - Published Version Available under License Creative Commons Attribution. Download (8MB) | Preview |
|
![[img]](https://real.mtak.hu/229587/7.hassmallThumbnailVersion/graphicalabst.jpeg)
|
Text (graphical abstract)
graphicalabst.jpeg - Published Version Download (83kB) | Preview |
Abstract
Research on rhenium complexes containing fac-tricarbonyl fragments has been on the rise in recent decades. Some complexes of this type exhibit advantageous properties that can be utilized in diagnostic and therapeutic applications. Herein, we report on the synthesis, structural characterization, solution speciation, and biological activity with mode of action studies of a new fac-tricarbonylrhenium(I) complex with pyrithione ligand fac-[Re(CO)3(pyrithionato)(benzonitrile)] (4). In an attempt to prepare a stable rhenium(I) complex with a pyrithionato ligand, several synthesis procedures were investigated and various products were discovered. In solution, the monodentate benzonitrile ligand can be replaced by a coordinating solvent molecule; however, the carbonyl ligands and pyrithione remain bound to the rhenium center. Complex 4 exhibited strong cytotoxic and antibacterial activity and effectively inhibited the growth of the HSV-2 virus. Additionally, complex 4 was also able to inhibit the cathepsin B enzyme (both its endo- and exopeptidase activities). In silico experiments confirmed that complex 4 can interact with cathepsin B near its active site, which may contribute to reduced enzymatic activity.
| Item Type: | Article |
|---|---|
| Additional Information: | Funding Agency and Grant Number: European Cooperation in Science and Technology [P1-0175, P4-0127, J3-3071, P1-0208]; Slovenian Research and Innovation Agency (ARIS) grants: a Junior Researcher Grant [I0-0022, CA21145]; ARIS (Infrastructure programme); COST (European Cooperation in Science and Technology); Hungarian Academy of Sciences [TKP2021-EGA-32]; Slovenian Academy of Sciences and Arts [TKP2021-EGA]; Ministry of Culture and Innovation of Hungary from the National Research, Development and Innovation Fund Funding text: This research was funded by the Slovenian Research and Innovation Agency (ARIS) grants: a Junior Researcher Grant to Uros Rapus, Grants P1-0175 and P4-0127 to Janko Kos, J3-3071 to Ana Mitrovic, and P1-0208 to Stanislav Gobec. The authors acknowledge the support of the Centre for Research Infrastructure at the University of Ljubljana, Faculty of Chemistry and Chemical Technology, which is part of the Network of Research and Infrastructural Centres UL (MRIC UL) and is also financially supported by ARIS (Infrastructure programme No. I0-0022), for the use of the Supernova diffractometer. This article is also based upon work from COST Action EURESTOP, CA21145, supported by COST (European Cooperation in Science and Technology). The authors thank the mobility support for bilateral international research projects of the Hungarian Academy of Sciences and the Slovenian Academy of Sciences and Arts. Project No. TKP2021-EGA-32 has been implemented with the support provided by the Ministry of Culture and Innovation of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-EGA funding scheme. The funders had no role in the study design, data collection and analysis, or preparation of the manuscript. |
| Uncontrolled Keywords: | Antimicrobial agents, Degradation, Inhibition, Ligands, Peptides and proteins |
| Subjects: | Q Science / természettudomány > QD Chemistry / kémia |
| SWORD Depositor: | MTMT SWORD |
| Depositing User: | MTMT SWORD |
| Date Deposited: | 21 Nov 2025 18:04 |
| Last Modified: | 21 Nov 2025 18:04 |
| URI: | https://real.mtak.hu/id/eprint/229587 |
Actions (login required)
 |
Edit Item |