Gráczer, Éva and Battirossi, Elena and Bozó, Tamás and Altorjai, Áron Gellért and Pászty, Katalin and Harsányi, Laura and Greve, Johannes N and Pertici, Irene and Reconditi, Massimo and Di Donato, Nataliya and Kellermayer, Miklós and Bianco, Pasquale and Varga, Andrea (2026) The Baraitser–Winter Cerebrofrontofacial Syndrome Recurrent R196H Variant in Cytoplasmic β-Actin Impairs Its Cellular Polymerization and Stability. THE FASEB JOURNAL, 40 (1). No. e71386. ISSN 0892-6638 (print); 1530-6860 (online)
|
Text
The FASEB Journal - 2026 - Gráczer - The Baraitser Winter Cerebrofrontofacial Syndrome Recurrent R196H Variant in.pdf - Published Version Available under License Creative Commons Attribution. Download (6MB) | Preview |
|
![[img]](https://real.mtak.hu/231470/7.hassmallThumbnailVersion/fsb271386-toc-0001-m.jpg)
|
Text (graphical abstract)
fsb271386-toc-0001-m.jpg - Published Version Available under License Creative Commons Attribution. Download (345kB) | Preview |
Abstract
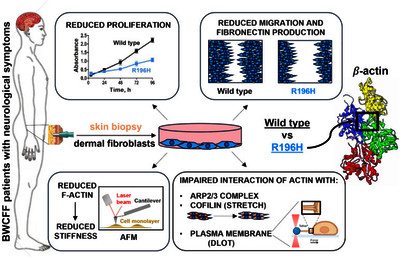
Variants in cytoskeletal actin encoding genes are associated with a broad spectrum of disorders, called non-muscle actinopathies. Among them, the Baraitser–Winter cerebrofrontofacial syndrome (BWCFF) displays the most severe symptoms, such as intellectual disability and epilepsy. We found that the BWCFF-associated R196H mutation results in reduced proliferation and migration of patient-derived fibroblast cells, and the latter is likely related to decreased fibronectin expression. The mutation causes a 50% drop in filamentous (F-) actin content, which is correlated with an approximately fourfold reduction in the stiffness of patient-derived cells probed with atomic force microscopy (AFM). We observed no significant defects either in the organization of the cellular actin cytoskeleton, analyzed by superresolution (STED) microscopy, or in the structure of purified filaments, explored with AFM. On the other hand, the more parallel orientation of the mutant actin bundles might be caused by the perturbed interaction of actin with the Arp2/3 complex. Manipulating the cells by mechanical forces through the application of the dual laser optical tweezers (DLOT) technique suggests that the mutation weakens the attachment of cytoskeletal actin to the plasma membrane. Inducing dynamic reorganization of actin by uniaxial stretching revealed that the interaction of cofilin with actin is also weakened by the mutation. Thus, the impaired function of actin to form filaments and interact with either cofilin or the Arp2/3 complex may result in the malfunction of dendritic spines, and the reduced cellular proliferation and migration might account for the lissencephaly phenotype of patients.
| Item Type: | Article |
|---|---|
| Uncontrolled Keywords: | actin cytoskeleton , atomic force microscopy , Baraitser–Winter cerebrofrontofacial syndrome , cofilin , optical tweezers , patient-derived fibroblasts |
| Subjects: | Q Science / természettudomány > QP Physiology / élettan |
| Depositing User: | Andrea Varga |
| Date Deposited: | 06 Jan 2026 09:05 |
| Last Modified: | 06 Jan 2026 09:05 |
| URI: | https://real.mtak.hu/id/eprint/231470 |
Actions (login required)
 |
Edit Item |